Trials & active studies
|
AZTEC
This trial is being led by Professor Sailesh Kotecha based at Cardiff University with Dr Berrington (Newcastle) as a co-investigator, and is an RCT exploring the effect of Azithromycin. Although advances in the treatment of premature babies has improved substantially over the last thirty years, many babies are still at risk of developing the lung disease called Chronic Lung Disease (CLD). Studies show that that both inflammation and the presence of microbes can increase the risk for developing CLD. AZTEC will target both the lung inflammation and the lung microbe called Ureaplasma and aims to determine if 10 days treatment with azithromycin reduces the rates of CLD. |
|
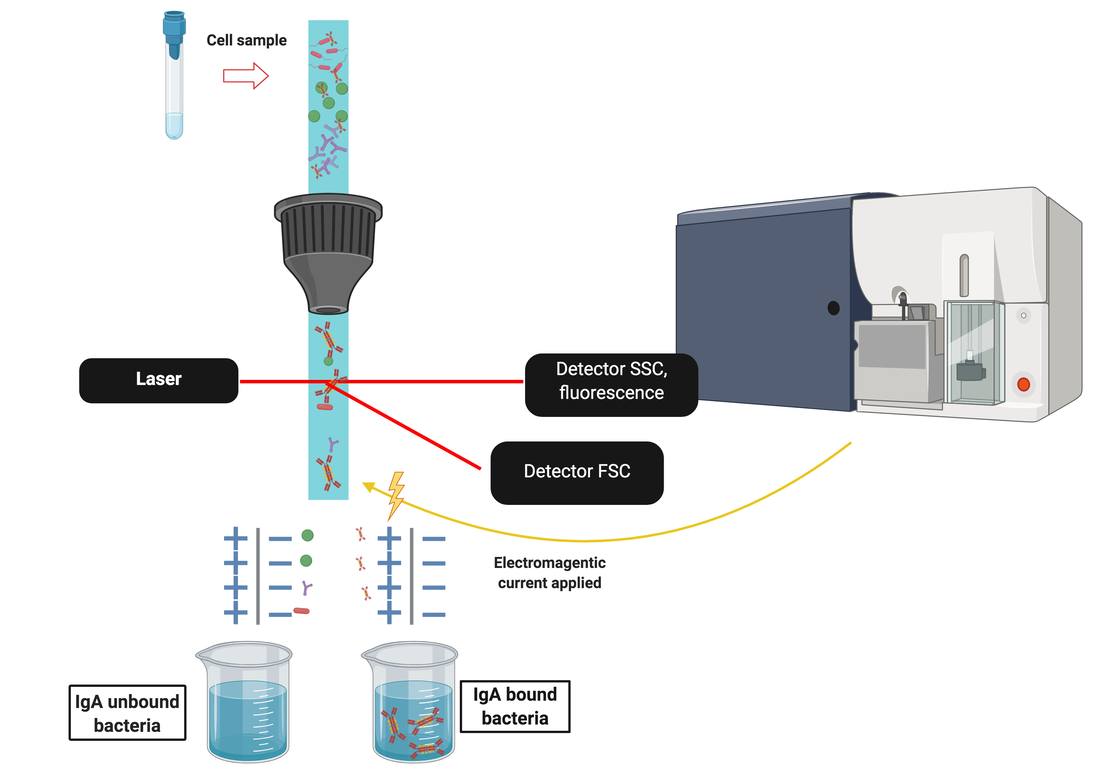
IGUANA - Immunoglobulin A and Neonatal Adaptation
Infants who are born preterm miss out on the essential transfer of immunoglobulins via the placenta. These infants therefore rely on maternal sIgAm (maternal secretory IgA from milk) from breast milk to provide immune protection in the first few weeks of life, until they begin to make their own intrinsic immunoglobulin via plasma cells. Breast milk is made up of almost all IgA (80%) with a smaller percentage being IgG and IgM. The mammary gland secretes sIgA into milk via pIgR as in the gut. Indeed IgA+ plasma cells in the breast tissue have originated in the GALT and migrated to the breast via the lymphatic system and blood, as directed by the chemokine CCL28. As such, the intestinal organisms that drive the strongest IgA responses in the mother shapes the sIgA secreted by the maternal mammary gland B cells. This work is being led by Dr Claire Granger, Dr Janet Berrington and Dr Chris Lamb (Newcastle University). |
|
Bacteriophage in Breastmilk Study (BiBS)
BiBS is exploring how certain viruses transmit from mothers breastmilk into preterm infants. Most people may think viruses cause infections and are best avoided, but there are certain viruses that infect (live inside) bacterial cells - these are called bacteriophages and all of us carry several trillion of these in our bodies! We think bacteriophages may be transmitted from mothers to babies and these may be important in 'shaping' the types of bacteria in the gut of preterm infants. We want to understand what these phages do, and whether they might be involved in benefiting the baby, perhaps by controlling the bacteria that cause sepsis or NEC. This work is being led by Dr Darren Smith from Northumbria University and is funded by ACTION MEDICAL RESEARCH. |
|
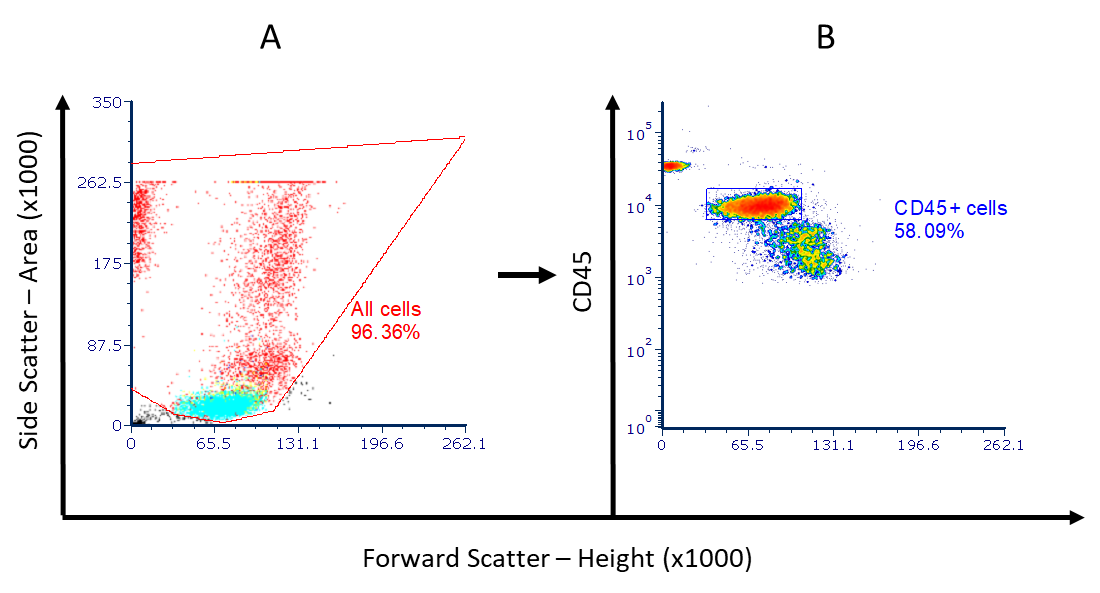
T cell development in Preterm Infants
T cells are essential in mediating a response to infections but few studies have explored T cell development after preterm delivery, and as part of INDIGO we are exploring T cell development. The majority of circulating T cells display a naïve phenotype but are still able to initiate a functional cytokine response similar to that seen in term infants. T-cells from preterm infants display a skewed T-helper 2 response and have an increased population of tolerogenic cells, suggesting that a range of other factors e.g. nutritional and other exposures, may impact on host response to infection. Innate and adaptive T cell populations congregate at mucosal surfaces where they interact with gut mucosal bacteria, offering a potential opportunity for modulation by pre/probiotics. This work is being led by Dr Tom Sproat and Professor Sophie Hambleton. |
|
INDIGO
The Interactions between Diet and Growth Outcomes (INDIGO) study explores how human milk affects gut bacteria. Mothers own breast-milk (MOM) is best and results in better outcomes such as fewer infections, but many mothers do not produce enough milk so either donor human milk (DHM) or a cow's milk formula are used to make up the 'shortfall'. Both MOM and DHM require fortification with additional nutrients in order to support optimal growth. This currently requires the use of a ‘fortifier’ derived from cow’s milk, but new milk supplements made only from donor human milk are now available. The pattern of gut bacteria in early life is linked to outcomes including infections and allergies, and this is especially important in preterm infants. The aim of this study is to compare two diets in very preterm infants and study the effect on the pattern of gut bacteria and chemicals (metabolites) in stool and urine, as well as growth and body composition. |
|
MAGPIE
Mechanisms Affecting the Gut of Preterm Infants in Enteral feeding trials. This was a mechanistic study nested within the ELFIN trial. We recruited 480 infants from 12 different NICUs in the UK. The study was conducted in close collaboration with ELFIN but was separately funded by the Efficacy and Mechanistic Evaluation (EME) stream of the NIHR. |
|
ELFIN
Enteral Lactoferrin in Neonates was a placebo controlled RCT and completed recruitment of 2204 babies in September 2017. It examined the effects of supplemental enteral lactoferrin on late onset sepsis and NEC and recruited premature infants from 30 large UK NICUs. The results were published in the Lancet in 2019 and are available here. ELFIN was funded by the Health Technology Assessment (HTA) stream of the NIHR. |

|
|
SIFT
The Speed of Increasing milk Feeds trial was an RCT comparing 18ml/kg to 30ml/kg/day increases in the rate of milk feeds. The primary outcome was neuro-developmental status at 2 years corrected age. SIFT recruited ahead of schedule and completed enrollment of 2804 babies in June 2015 making it the largest interventional feeding study in preterm infants every conducted worldwide. The results were published in the New England Journal of Medicine in 2019 and are available here. ELFIN was funded by the Health Technology Assessment (HTA) stream of the NIHR. |

|