We are a broad based research group and many of our areas overlap. We have grouped these into a few areas including
- Cohort studies (see below)
- Trials
- NEC, gut "omics" and enteroids
- Motor function, therapy and outcome
- Qualitative studies
Newcastle Neonatal Research Group - cohort studies
|
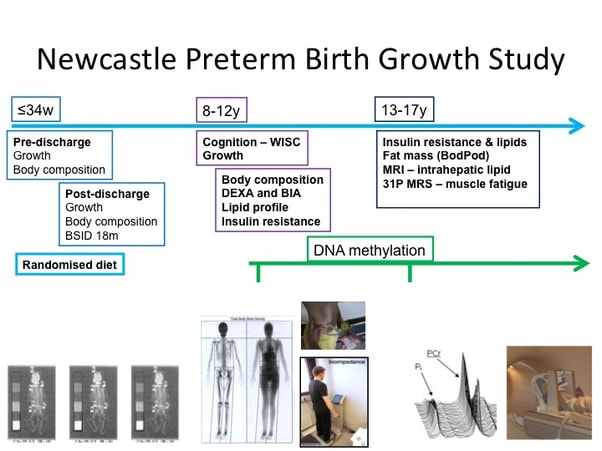
The Newcastle Preterm Birth Growth Study (PTBGS) was formed as a follow on study from two trials conducted in Newcastle in the late 1990's in preterm (24–34 weeks gestation) low birth-weight infants. 247 infants were recruited prior to hospital discharge. Infant follow-up included detailed measures of growth, nutritional intake and body composition (Dual X Ray Absorptiometry, DXA) along with demographic data until 2 years corrected age. Developmental assessment was performed at 18 months corrected age & cognitive assessment at 9–10 years of age.
Growth, body composition (DXA), blood pressure and metabolic function (insulin resistance and lipid profile) were assessed at 9–13 years of age, and samples obtained for epigenetic analysis. In the most recent study, we used established techniques and novel metabolic biomarkers and correlated them with lifestyle factors such as physical activity and dietary intake. We assessed growth, body composition (BODPOD™), insulin resistance, daily activity levels using Actigraph™ software and used magnetic resonance spectroscopy (MRS) to assess mitochondrial function and intra-hepatic lipid. |
|
The Feeding in Late and Moderately preterm Infants Nutrition and Growth Outcomes (FLAMINGO) study was started in 2017 and recruited over 180 preterm infants born between 32 and 36 weeks gestation. The study measures growth, body composition (DXA) and nutritional intakes until 2 years age and enrols infants who are breast or formula milk fed. We collect mouth swabs and stool samples to explore patterns of gut and oral bacteria (microbiota), and use questionnaires to assess feeding behaviours, duration of breast feeding and timing on introduction of complementary foods (weaning).
|
|
Late and Moderately Preterm infants (LMPT) represent around 6-7% of all births and are much more prevalent than very preterm births. Some are born early because of pregnancy complications, but some are spontaneous. The majority do not need admission to intensive care. However, there is evidence to show that metabolic and cognitive outcomes are worse for these infants, especially if they do not receive breast-milk. We want to understand how early growth affects later outcomes, and the factors associated with breast-feeding duration that may allow us to better support mothers.
|
|
SERVIS study - this study forms the basis of the samples stored as part of our Great North Neonatal Biobank. Since 2011 we have recruited over 750 preterm infants born <32 weeks gestation who received care on the NICU in Newcastle. We have analysed stool, urine, blood, breastmilk, and residual gut tissue using microbiomic, metabolomic, transcriptomic, biochemical and genetic methods. Much of this worked is undertaken in the Stewart Lab. We have permission to keep in contact with families so these infants can form the basis of a future cohort to track into later life.
|